States
Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Texas, Utah, Vermont, WisconsinEcosystem
River/streamInvasive Carp in the United States
Management of invasive carp populations is one of the biggest conservation challenges facing the United States today. Assessing the reproductive potential of grass carp and black carp in the wild can help fisheries managers determine control methods for invasive carp species. Grass Carp and Black Carp can be important biological tools for managing closed water systems. Grass Carp are voracious herbivores and can be stocked in ponds or lakes to reduce unwanted vegetation that may clog boat motors or affect recreational activities. Black Carp feed on snails, which often carry parasites, and can be used in aquaculture to reduce parasitic infections in farm-raised fish. The problem arises when stocked fish escape into waters where they could reproduce and compete with native species or impact endangered freshwater mussel and snail populations.
Creation of Sterile Stocks
A solution to prevent stocking of reproductive Grass Carp and Black Carp into the wild is to create a sterilized carp for stocking purposes. Grass Carp producers create sterile stocks of both carp species by treating the eggs to produce a triploid fish. Triploid fish have extra copies of DNA in each cell, which causes errors in the reproductive process, thus fish are functionally sterile and cannot reproduce. Many states require that all stocked Grass Carp be triploid for stocking purposes. Since the mid-1980s the U.S. Fish and Wildlife Service's National Triploid Grass Carp Inspection and Certification Program has permitted and certified triploid shipments to states where non-reproductive triploid Grass Carp stocking is allowed. Black Carp are prohibited in most states, but some states permit stocking of triploids by fish farmers to control snail-borne parasites in fish production ponds.
Determination of Ploidy Status
Since 2013, the La Crosse Fish Health Center has been leading the DNA Ploidy Analysis of Grass and Black Carpprogram for the Nation after the science was clearly established, and the technology was transferred from the Departments of Interior’s research agency, U.S. Geological Survey Invasives Species Research Program.
When a Grass Carp or Black Carp is captured in the wild, eyeball or blood samples may be collected and sent to the Center for ploidy determination. The La Crosse Fish Health Center uses flow cytometry to determine the ploidy status of carp samples. A sterile, or triploid, fish has three copies of nuclear DNA, whereas a diploid, or naturally produced fish, has only two copies. Nuclei from a triploid fish contain more DNA than diploid nuclei because of the increased nuclear DNA content. A flow cytometer measures fluorescence from stained DNA and compares samples to a known standard.
Results are collected in the USGS Nonindigenous Aquatic Species Database which catalogs, assembles data, and can generate spatially referenced accounts of the invasive carp in maps. Knowledge of the ploidy status of wild-caught invasive carps can help focus control efforts where needed. All information is available to the public.
Contact our Ploidy Analysis Coordinator: sara dziki
Relevant Publications
Jenkins, J.A. and R.G. Thomas. 2007. Use of eyeballs for establishing ploidy of Asian carp. North American Journal of Fisheries Management 27:1195-1202.
Jenkins, J.A., M.D. Chauvin, D. Johnson, B.L. Brown, J. Bailey, A.M. Kelly, B.T. Kinter. 2019. Defensible standardized ploidy assessments for Grass Carp (Ctenopharyngodon idella, Cyprinidae) intercepted from the commercial supply chain. Journal of Great Lakes Research 45: 371-383.
Directions for Sample Collection
Please include the Ploidy Analysis Data Collection Form to your shipment.
Eyeball Removal, Sample Preparation, and Shipping Procedures for Ploidy Analysis
Materials
- Forceps; scalpel; blunt or curved scissors
- Permanent marking pen
- 50‐100 ml plastic containers with leak‐proof screw top cap
- Sealable plastic bags to fit several 50‐100 ml containers
- Contact lens solution or saline (0.8‐1.0% NaCl in DI water) (1 g NaCl per 100 ml of DI water)
- MS‐222 or other means of euthanasia
Cooler or insulated container with ice packs, packing tape to seal cooler
NOTE: Contact the La Crosse Fish Health Center if you have questions regarding the materials needed or to request assistance with preparing a kit for sample preparation and shipment.
Eyeball Sample Preparation for Overnight Shipment – Do Not Freeze:
- Euthanize fish according to American Veterinary Medical Association standards.
- Label a small, plastic container with collection date, species, and sample number (e.g. 25MAR22, black carp, #12)
- Use forceps to hold the eyeball steady. Taking care not to puncture the eyeball, insert scalpel blade between the eyeball and socket wall with the blade pointed outward toward the socket wall. Cut around the circumference of the eyeball until the eyeball moves freely in the socket.
- Use the blunt or curved scissors to reach behind the eyeball and cut the optic nerve. Once the optic nerve is cut, you should be able remove the eyeball and trim off any excess tissue.
- Remove the other eyeball and place both eyeballs in the labeled container.
Pour contact lens solution or saline into the labeled container until full. Both eyeballs should be completely immersed. Close lid tightly. Maintain at 4 to 8°C. Do Not Freeze.
Contact Sara on day of collection or as soon as possible to make Overnight Shipping arrangements. If fish are collected live, blood samples may be collected 1:1 in Acid Citrate Dextrose (ACD) as an alternative to eyes. Keep samples on ice and ship overnight.
Shipping
- Pack samples in a Ziploc bag to prevent leakage and then enclose in a sealed, insulated cooler with ice packs to maintain 4 to 8°C. Include collection data form with package. Tape lid securely.
- Ship priority overnight to La Crosse Fish Health Center (address on data collection form).
- Email confirmation of shipment and tracking numbers to sara_dziki@fws.gov; include digital images and GPS sampling location with this email.
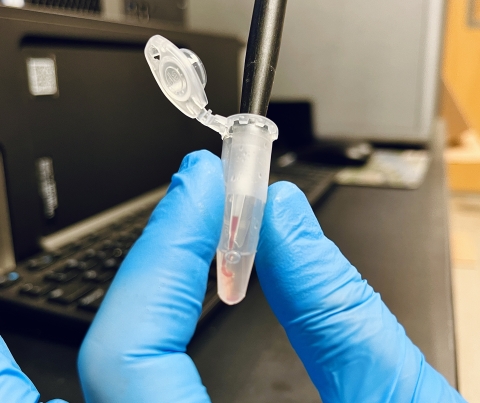
Blood Collection for Non-Lethal or Lethal Ploidy Analysis (Fish must be live at time of collection):
- Anesthetize fish appropriately for handling or euthanization.
- Using a 3 ml syringe with a 21G needle attached, insert needle through ACD stopper and draw up a few drops of Acid Citrate Dextrose into the syringe. Set blood collection tube aside.
- Holding the plunger, insert the needle into the caudal vein at the tail (red ‘x’ is almost guaranteed) or at an angle (upwards) just below the lateral line (blue star) until you find the vein. If you hit the spine, pull the needle out slightly (about 1mm) and reinsert just below that spot. The vein lies directly below the spine, so you should hit it promptly now.
- Watch the base of the needle, when you hit the vein you will see a burst of blood into the syringe, stop moving and allow the blood to collect in the syringe until you have 1-2 mls. You may pull on the plunger with gentle pressure to collect enough blood. (If all you can get is ½ ml, that’s fine)
- When you have enough, remove the needle.
- If it is a non-lethal sample, put pressure on the spot to encourage clotting.
- Re-insert the needle through the rubber stopper of a vacutainer containing the remaining 0.5 to1 ml of ACD (acid citrate dextrose).
- Depress the plunger to be sure all of the sample is dispensed. Keep cool (4-8C).
- Label tube and ship on ice priority overnight to La Crosse Fish Health Center (555 Lester Avenue Onalaska, WI 54560).
Alternative method using heparinized hematocrit tube: Make a cut at the tail. Collect blood in a heparinized tube to draw blood. Remove stopper from ACD vacutainer. Drop the tube into the ACD solution. Replace stopper, keep on ice and ship overnight to La Crosse Fish Health Center.